Hydrogen (H₂), the lightest and most abundant element in the universe, is widely regarded as an ideal clean energy source due to its odorless, non-toxic nature and its clean combustion that produces only water. With an energy density three times that of fossil fuels, hydrogen holds immense potential for applications in power generation, heating, transportation, and industrial raw materials.
As a strategic key to achieving carbon neutrality, the large-scale application of hydrogen energy has long awaited breakthroughs. However, large-scale hydrogen energy application faces two key challenges: high production costs and storage/transportation difficulties. Only by achieving safe, efficient, and low-cost hydrogen storage and transport can it be integrated into existing energy systems for widespread use.

Recently, a research team led by Professor DingMa from the College of Chemistry and Molecular Engineering at Peking University, in collaboration with other scientists, published two groundbreaking researchworksin Nature and Science, the world's top scientific journals. Through innovative catalyst design and a novel hydrogen production pathway, they achieved high-efficiency hydrogen production with zero carbon emissions, contributing pioneering Chinese ingenuity to the global transition to green energy.
Dual Breakthrough: Catalyst Innovation and Hydrogen Production Reform
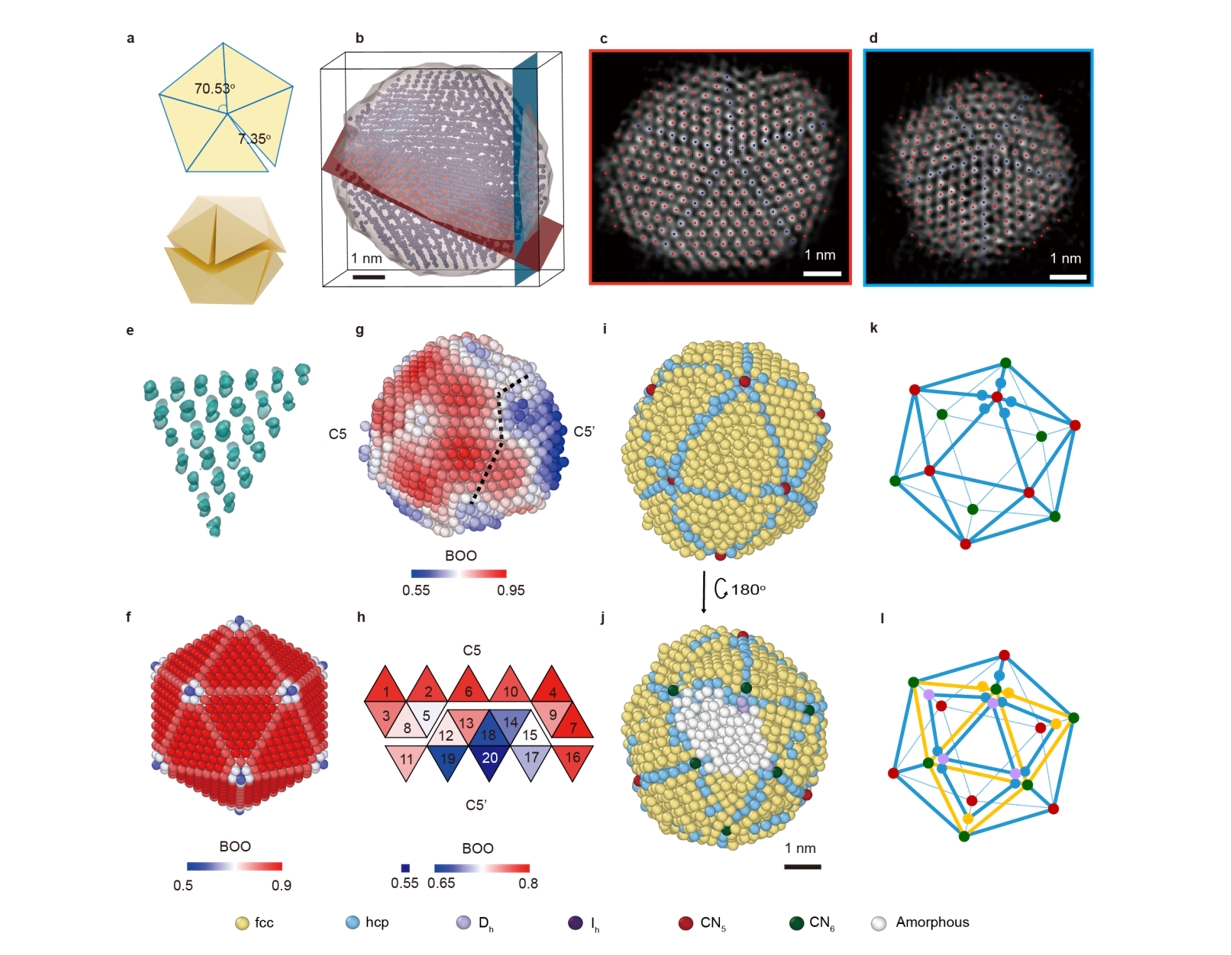
Catalysts play a crucial role in hydrogen production, yet traditional catalysts suffer from rapid deactivation and short lifespans. In their Naturepaper, Professor DingMa's team introduced a pioneering strategy that employswell-dispersed inertoxide nano-overlaysto protect thecatalyst—successfully overcoming the long-standing issue of catalyst instability and achievinga new international benchmark for similar reactions.
"Not only have we developed a highly efficient and stable new catalyst, but more importantly, we have established a universal strategy for designing and improving stable high-activity catalysts. This approach can be applied to manyothercatalytic processes." said Dr. ZiruiGao,the first author of the article.
In parallel, their Science study focused on an innovative ethanol-water partial reforming pathway, introducing revolutionary "zero-carbon hydrogen production" technology. Under mild conditions of approximately 270°C, the team achieved selective conversion of ethanol and water molecules, precisely yielding hydrogen and acetic acid, without releasingcarbon dioxide. This breakthrough significantly reduces energy consumption and product separation costs, enhancing the economic viability and feasibility of hydrogen production while opening new pathways for biomass resource utilization.
Both studies leveraged cutting-edge characterization techniques, allowing researchers to visualize atomic structures on catalyst surfacesand reconstruct their three-dimensional configurationswith advance electron microscopy techniques. These advancements are expected to have profound implications for catalyst research and development.
Bright Prospects for the Hydrogen Energy Industry
"Our goal is not only to push the frontiers of science but also to provide practical solutions to energy and environmental challenges." remarked Professor DingMa. His team’s pioneering research has garnered extensive interest in the academic community and further provided strong support for the real-world application of clean energy technologies.
Currently, hydrogen energy stands at a pivotal stage of technological innovation and industrial expansion. Looking ahead, as the reliance on precious metals diminishes and more economical, high-performance catalysts emerge, the cost of hydrogen production is anticipated to decline progressively, paving the way for its accelerated global integration. Hydrogen energy is poised to become the most shining star in the global clean energy system, making invaluable contributions to achieving carbon neutrality and a sustainable future.